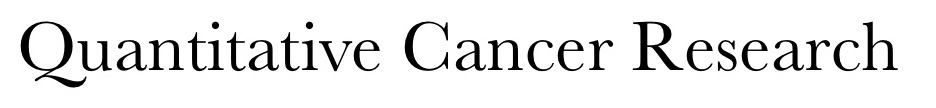|
||||||||||
| Home | Research | People | Recent Publications | Courses | Contact us | |||||
Research Overview
As a group of genetic diseases, cancer presents some of the most challenging problems for basic scientists, clinical investigators, and practitioners. In order to design treatments that are capable of specifically targeting the invasive cancer cells that drive malignant tumor growth, it is necessary to understand the mechanisms by which these cells initiate angiogenesis, enhance their motility, relax adhesive cellular bonds and penetrate normal tissue. Due to the inherent complexity of the many interconnected physiological processes involved in tumor initiation, new blood vessel formation, and cell invasion, conventional experimental approaches alone are often unable to penetrate to the core of these issues. Further, given the multi-scaled pathophysiology involved, it is becoming ever so important for cancer research to make use of cross-disciplinary, systems science approaches, in which innovative computational cancer models play a central role. Our research is aimed at combining mathematical modeling, numerical simulation, and carefully designed experiments to develop a comprehensive and predictive framework for better understanding tumor development and for improving cancer treatment. 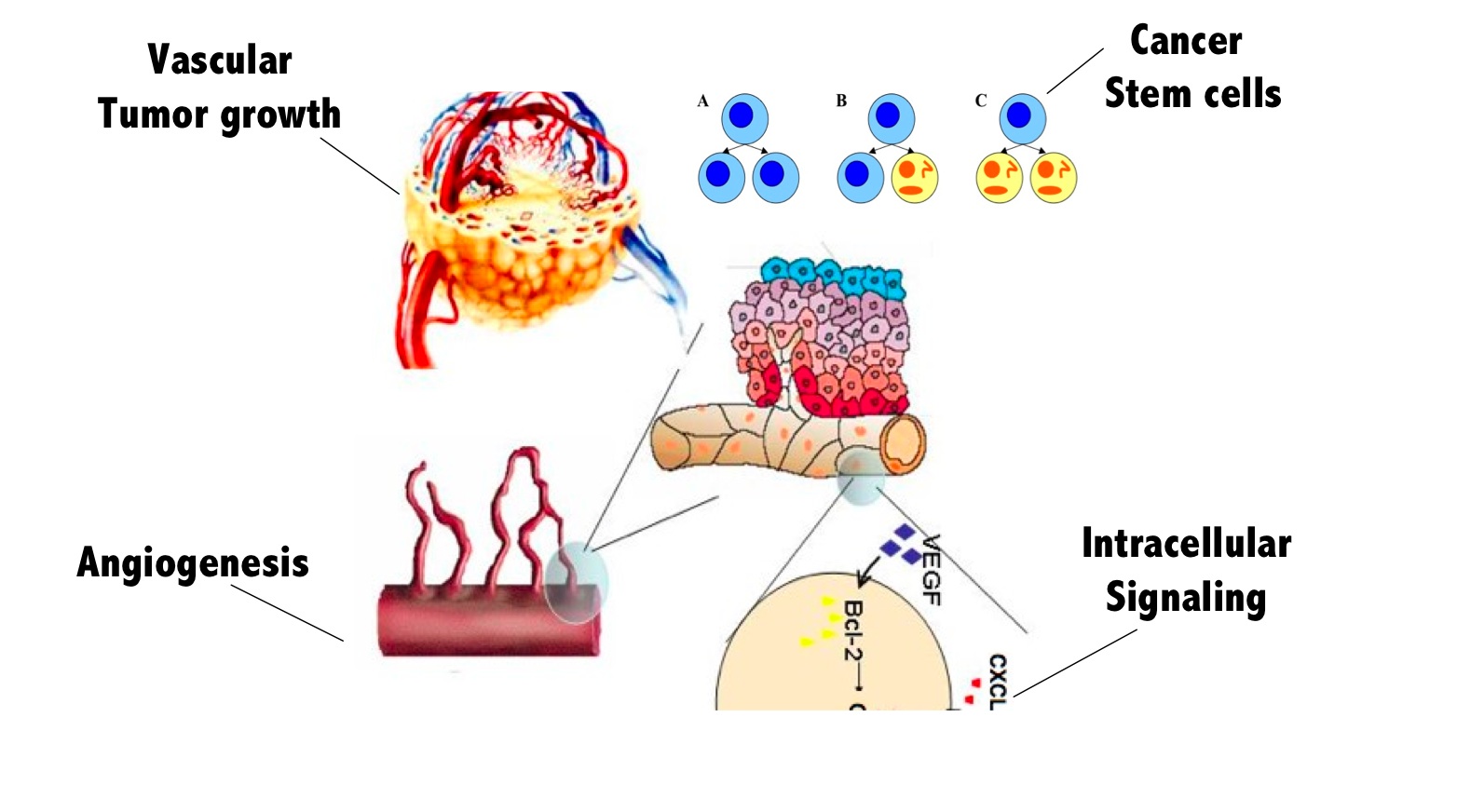
Research Areas 1. Molecular Pathways Associated with Intratumoral Angiogenesis 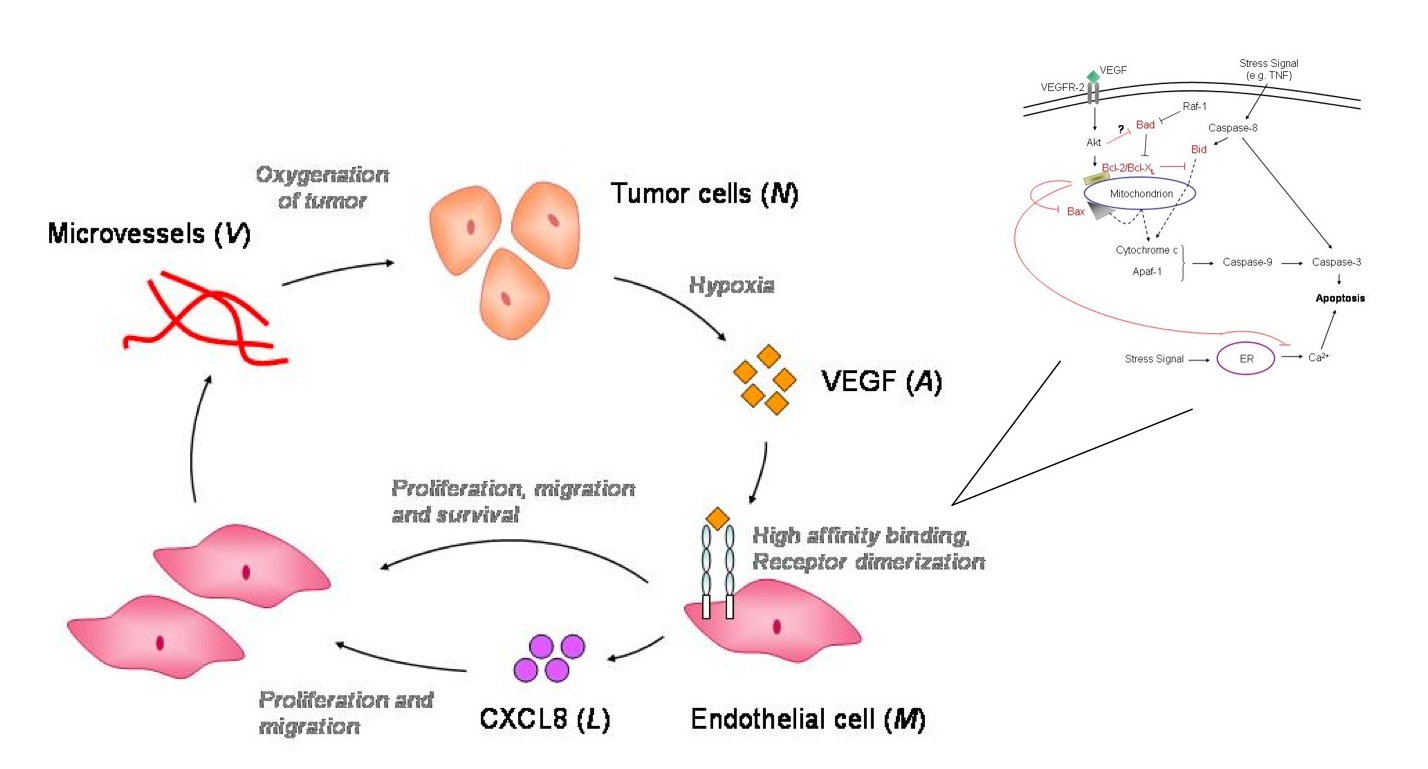 Recent experiments show that vascular endothelial growth factor (VEGF) is the crucial mediator of downstream events that ultimately lead to enhanced endothelial cell survival and increased vascular density within many tumors. The newly discovered pathway involves up-regulation of the anti-apoptotic protein Bcl-2, which in turn leads to increased production of interleukin-8 (CXCL8). The VEGF - Bcl-2 - CXCL8 pathway suggests new targets for the development of anti-angiogenic strategies including short interfering RNA (siRNA) that silence the CXCL8 gene and small molecule inhibitors of Bcl-2. We are building mathematical models that connect the molecular events associated with VEGFR2 dimerization and intracellular signaling with temporal changes in endothelial cell proliferation, migration and survival and relating these processes to tumor growth and vascular structure.
2. Cell-Tissue Interactions Associated with Tumor-Induced Angiogenesis 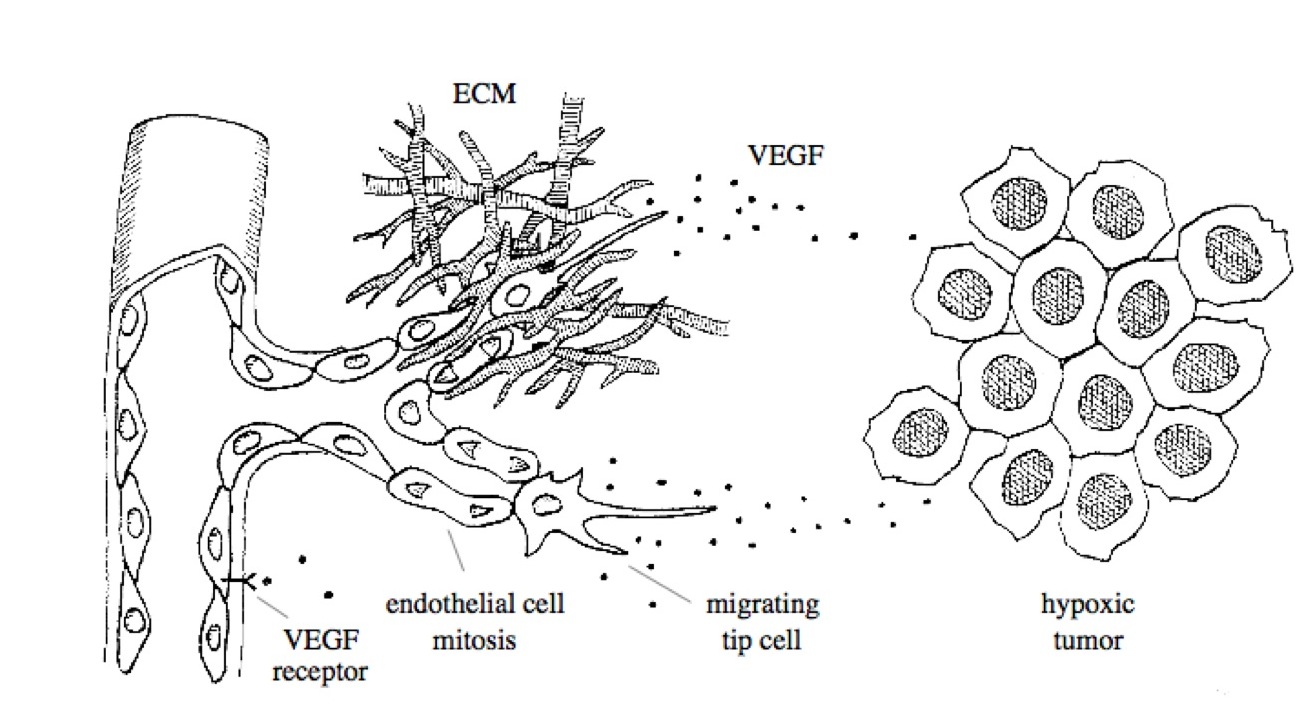 The emergence of new blood vessels in response to a tumor is the result of complex interactions between endothelial cells and extracellular matrix (ECM); with equally important contributors being the chemokines, growth factors, and adhesion molecules that stimulate and connect the cells and the stroma. Detailed knowledge of each individual system component is not sufficient to understand their collective dynamics. Achieving an integrative understanding of the molecules, cells, and tissue structures that coordinate to form new blood vessels in response to tumors is the overarching goal of this research. Our modeling strategy brings together continuous and discrete approaches to derive and validate a multiscale, cell-based model of tumor-induced angiogenesis.
3. Tumor Heterogeneity and Cancer Stem Cells 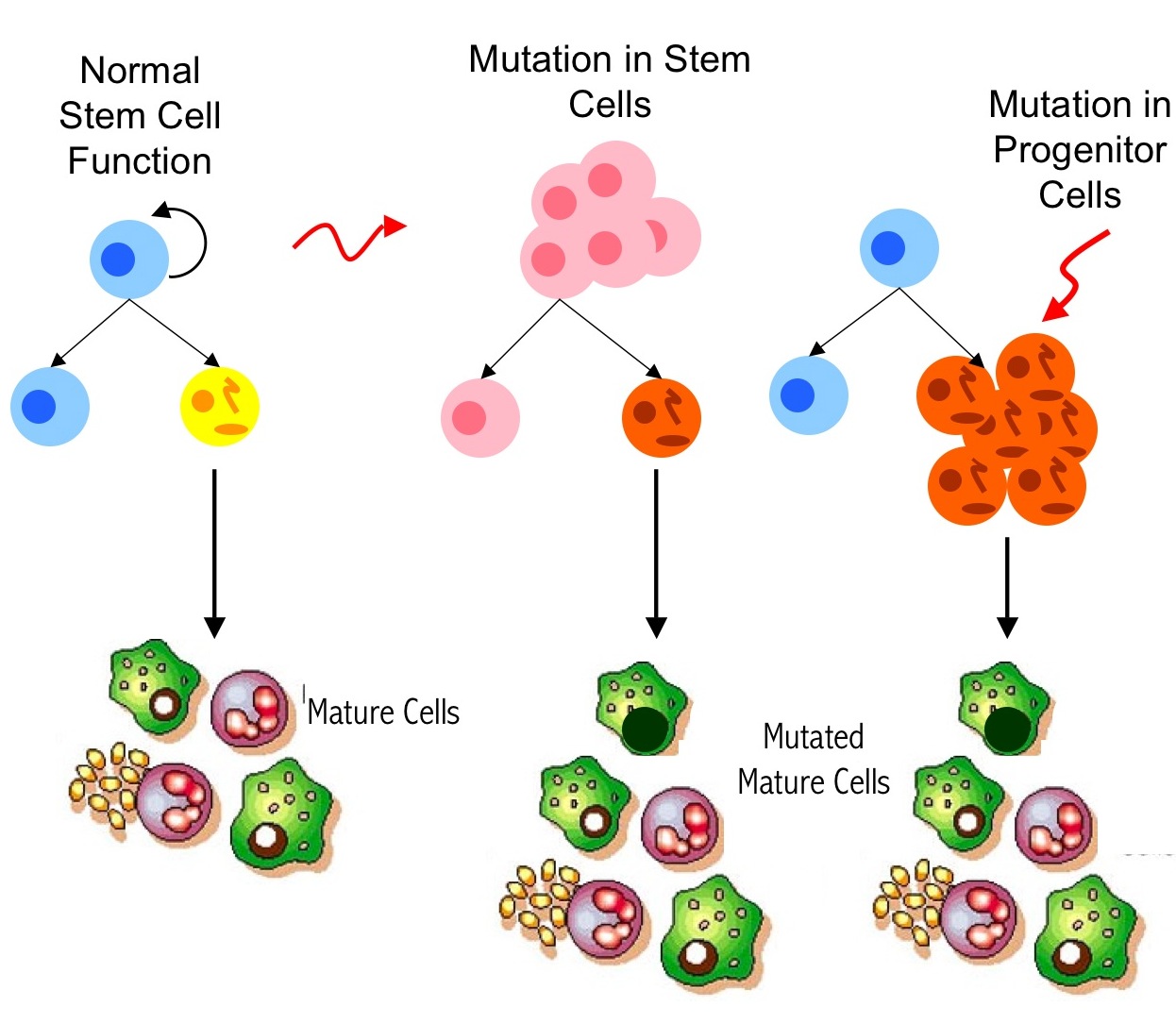 In recent years, exciting advances in cancer research have led to the isolation of select cells, cancer stem cells, that have the capability of initiating and sustaining tumor growth. These transformed stem cells are being identified within an increasing variety of malignancies and are believed to play a key role in tumorigenesis and disease progression. We are interested in developing and utilizing quantitative measures to improve current methods of stem cell identification and to understand their role in tumor growth, heterogeneity, and resistance to therapy.
|
||||||||||
| Mathematical Biology Research Group Home | Department of Mathematics Home | |||||||||